Experiment of The Month
Absolute Zero
The MU Physics Department does not claim to have invented these labs; their origin is currently unknown to us. Our labs do not have written instructions. In keeping with this spirit, the description given here will be brief and general. The intent is that each performance of the lab will be unique; in each nature will reveal a slightly different face to the observer
This laboratory determines the temperature of absolute zero using materials available in the kitchen. It was developed by Dr. Miziumski for use in Physics 103, the course for students not majoring in science.
The most sophisticated piece of equipment is an Erlenmeyer flask, sealed with one-hole rubber stopper, fitted with a glass tube and hose. The flask is used as a constant pressure gas thermometer. The gas used is simply air, and the pressure is simply atmospheric pressure. When the temperature changes, the volume of the gas changes, and the volume change is used a measure of the temperature change. The novel aspect of this exercise is the method of determining the volume change
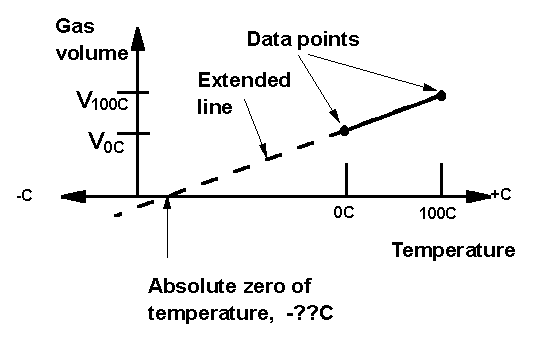
We define the Celsius temperature scale in the usual way, so that 0C is at the freezing point of water, and 100C is at the boiling point. The gas in the thermometer can, in principle expand without limit, indicating that there is no upper limit on temperature. On the other hand, the minimum volume of the gas is zero, and this suggests that there is a minimum temperature; that nothing can be colder than the temperature at which the volume of the gas thermometer is zero. That temperature is called "absolute zero."
Since we have defined temperature to be proportional to the volume, we know that a plot of gas volume versus temperature is a straight line, with positive slope. If we can establish that straight line near room temperature, then with a ruler we can extend it down, until it reaches the temperature at which the volume is zero. That temperature is absolute zero. The hardest part of this lab is to prevent the students from immediately labeling that temperature as -273C. The point of the exercise is, of course, that we can establish experimentally our own Celsius temperature value for absolute zero.
The goal here is to keep a constant amount of gas in the flask, at constant pressure, and observe the change in volume of that amount of gas as the temperature change. The amount of gas used is defined as follows: It is the gas which fills the flask completely when the temperature is 100C (boiling water). We establish this amount of gas in a way which illustrates the effect of heat on the gas:

The flask is sealed with the stopper, and the rubber tube from the flask is immersed in salad oil, as shown above. The flask is heated in boiling water (we heat our water in a "FryDaddy" fryer). As the air is heated, it expands, and bubbles out through the oil. This gives students a first look at the expansion of a gas when it is heated. If the tube is held near the surface of the oil, then the pressure at the mouth of the tube is one atmosphere; and so is the pressure in the flask. Once the tube has stopped passing air bubbles, we know that the temperature of the gas is at 100C, and has stopped expanding. Then, keeping the tube under the oil at all times, we go to the second stage of the experiment.
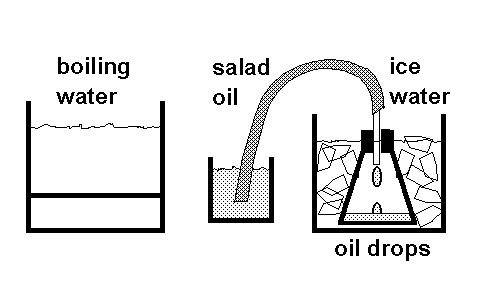
The flask is transferred to an ice water bath. The air cools, and the atmospheric pressure in the room forces salad oil up through the tubing into the flask. When the oil stops dripping, (with the level of the salad oil IN THE SMALL BEAKER at the same height above the table as END OF THE TUBE IN THE TOP of the flask, so we know the pressure inside is one atmosphere) we know that the temperature of the gas is at 0C, because the gas has stopped shrinking from the cold.
Now we need to find the two gas volumes: at 100C and 0C. The volume at 100C is found by filling an identical flask with water, and measuring the volume of that water. It is important not to allow water into the thermometer flask itself: A little bit of water vapor is big trouble, because it will go through a phase change during the experiment, and will fail to obey (even approximately) the ideal gas law.
Once these volumes are known, the plot can be made, and the student's own personal value for the temperature of absolute zero can be determined, just by reading it from the graph.
-
Contact Information
Contact Number: 717-871-4297
Email: physics@millersville.edu